REVIEW ARTICLE
Infection, infection control, and disinfectants in a challenging infection era
Philip G. Bowler*
Phil Bowler Consulting Ltd, Appleton, Warrington, UK
Abstract
Health care-associated infections inflict a huge clinical and economic burden on public health worldwide. Bacterial resistance to antibiotics continues to escalate, and antimicrobial stewardship initiatives have yet to make a major impact. Additionally, the ability of bacteria to evade environmental threats by living within a self-produced protective biofilm and/or producing resistant spores further challenges effective infection control. The current severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has also amplified the burden significantly. Amidst a particularly challenging infection era, the demand for meticulous infection control and prevention practices is paramount, a key component of which is the use of appropriate disinfectants that can combat a wide variety of microbial pathogens, including diverse forms of viruses and bacteria, particularly highly tolerant spore-forming and biofilm-forming microorganisms. This review addresses the advantages and disadvantages of commonly used disinfectants such as alcohols, hypochlorite, and quaternary ammonium compounds, together with oxidizing agents such as chlorine dioxide and peracetic acid, which are gaining increasing acceptance in routine infection control practices today. Given the increasing requirements for rapid-acting disinfectants that are effective against the toughest of microorganisms (e.g. spores and biofilm), are environmentally friendly, and remain active under diverse environmental conditions, emerging oxidizing agents warrant further consideration, particularly chlorine dioxide, which offers most requirements for an ideal disinfectant, including retention of activity over a broad pH range. Given the critical importance of infection control and antimicrobial stewardship in public health and health care facilities today, consideration of chlorine dioxide as a safe, selective, highly effective, and environmentally friendly disinfectant is warranted.
Keywords: infections; health care-associated infections; biofilms; infection control; disinfectants
Citation: Int J Infect Control 2021, 17: 21564 – http://dx.doi.org/10.3396/ijic.v17.21564
Copyright: © 2021 Philip G. Bowler. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for any purpose, even commercially, provided the original work is properly cited and states its license.
Received: 26 April 2021; Revised: 19 July 2021; Accepted: 15 August 2021; Published: 11 October 2021
Competing interests and funding: The author provides consultancy services to LifeClean International AB. Preparation of this manuscript was supported by LifeClean International AB, a provider of disinfectant solutions. The sponsor was not involved in the content of the manuscript.
*Philip G. Bowler, Phil Bowler Consulting Ltd, 18 Hartswood Close, Appleton, WA4 5QZ Warrington, UK. Email: phil.bowler@hotmail.co.uk
Infections inflict a major clinical and economic burden on public health on a global scale. Within an alarmingly short timeframe, SARS-CoV-2 has disseminated uncontrollably with devastating speed and effect, infecting approximately 230 million people and accounting for more than 4.7 million deaths at the present time. While SARS-CoV-2 wreaks havoc across the globe, a longer-term, underlying, and escalating threat of bacterial resistance to antibiotics continues. Those most seriously affected by SARS-CoV-2 requiring intensive care become more susceptible to serious bacterial infections that antibiotics are increasingly less effective against. This combination exacerbates the threat to human health.
Health care-associated infections (HCAIs), that is, those acquired while in a health care setting (e.g. hospital, clinic, and long-term care), have a huge impact on patient morbidity and mortality, and on costs to health care. Prevalent HCAIs include catheter-related blood stream infections, surgical site infections, urinary tract infections, and respiratory tract infections (1). A particular spore-forming bacterium, Clostridiodes (Clostridium) difficile, has been reported to be responsible for 5.6% of all infections within the National Health Service (NHS) in England (2), and for a ~$1.5 billion cost burden to US health care annually (1). In a recent analysis of the impact of HCAIs on cost to the NHS in England, Guest et al. estimated 834,000 HCAIs during the period 2016/2017, costing the NHS £2.7 billion and accounting for 28,500 patient deaths (3). This study highlighted the need for strict adherence to infection control practices and guidelines.
While the global impact of antibiotic resistance and more recently the SARS-CoV-2 pandemic are widely acknowledged, another largely unappreciated factor that contributes significantly to the spread of infection is biofilm. This review will expand on the implications of biofilm – what it is, how it contributes to HCAIs and antibiotic resistance, and why biofilm should be acknowledged and targeted in infection control practices.
In an era of continuing bacterial resistance to antibiotics and a viral pandemic, the demand for meticulous infection control practices is greater than ever. In this respect, disinfectants and disinfection procedures are critical, and it is extremely important that disinfectants can combat a wide variety of pathogens that cause such devastating infections – including different forms of viruses and bacteria, particularly spore-forming and biofilm bacteria, which are the most difficult to kill.
What are biofilm bacteria?
Biofilm is an extracellular matrix produced by bacteria to protect themselves from the outside world. Although bacteria are widely acknowledged as existing as actively dividing single cells (sometimes referred to as planktonic or vegetative cells), they much prefer to exist as surface-attached communities. Once attached to a surface (which could be a viable tissue surface or a non-viable surface), bacteria secrete an extracellular polymeric substance around themselves to provide protection from external environmental threats such as extreme temperature, desiccation, immune cells, and antimicrobial agents. This is biofilm, and it is the natural and prevalent form of bacterial life. Biofilm dominates all habitats on the Earth’s surface and has been reported to account for an estimated 80% of the approximate 1.2 × 1030 bacterial cell population (4). We see biofilm in everyday life: dental plaque on the surface of teeth, the scum in a blocked drain, and the slime in a vase of flowers are all examples of bacterial biofilm. Since the 1980s, the implications of biofilm in chronic diseases have been well-documented, and infections such as otitis media, catheter-associated urinary tract infections, and those associated with chronic, non-healing wounds are now recognized as biofilm infections (5, 6). In 2002, the United States National Institutes for Health reported that biofilms account for over 80% of human infections (7). Typically, biofilm is difficult to remove and protects bacteria from the effects of antimicrobial agents; the minimal bactericidal concentration (MBC) of bacteria protected by biofilm has been reported to be 100–1,000 times higher than that of planktonic (unprotected) bacteria (8). Consequently, biofilm infections invariably manifest as difficult-to-treat, persistent, and recurrent infections. More recently, viruses have been shown to exist within biofilm communities in flowing freshwater habitats (9), but in contrast to self-produced bacterial biofilm, viral biofilm forms via acquisition of matrix components from the infected host cell (10). The formation and existence of SARS-CoV-2 (COVID-19) biofilm has recently been hypothesized (10).
Biofilm in the health care environment
Aside from bacterial biofilm causing chronic infections, environmental biofilm is also a major concern in health care facilities. Given the ubiquity of biofilm, it will form and enable bacterial survival on non-viable surfaces such as toilet basins, sink units, drains, beds, and medical devices (e.g. endoscopes) over prolonged periods of time. A study conducted in German hospitals with high antibiotic consumption regularly detected antibiotic residues in toilets, sink siphons, and shower drains (11). Although flushing was shown to remove antibiotic residues, biofilm quickly reformed, and antibiotics were detected again. This study confirmed the ability of the biofilm to act as a reservoir for the accumulation of antibiotics in hospital sanitary units. Additionally, the transfer of antibiotic resistance genes between bacterial cells has been shown to occur 700 times more efficiently within biofilm than among free-living planktonic bacterial cells (12). Consequently, the presence of environmental biofilm in health care facilities is highly likely to enhance the spread of antibiotic resistance, in addition to facilitating bacterial survival and spread of infection (13).
Although biofilm grows most abundantly in wet conditions (i.e. wet surface biofilm), dry surface biofilm (DSB) is also a major concern in health care facilities. Biofilm containing antibiotic-resistant bacteria has been shown to persist for up to 12 months on equipment and furnishings in an intensive care unit, despite prior terminal cleansing involving detergent and bleach (14). In a UK study in three hospitals, DSBs were recovered from the surfaces of keyboards, patient folders, and hand sanitizing bottles, despite prior cleaning (15).
The ubiquity and implications of environmental biofilm within health care facilities and the criticality of effective biofilm control within infection control and prevention practices are clear.
Antimicrobial agents
Cleaning and disinfection are critical components in the control and prevention of HCAIs. Together with antibiotics and antiseptics, disinfectants are antimicrobial agents. The term ‘antimicrobial agent’ is broad-ranging and captures agents that can kill microorganisms (essentially bacteria, yeasts, fungi, and viruses) or prevent their growth. Antibiotics are primarily natural chemical substances produced by microorganisms to provide competitive advantage over other microorganisms. Antibiotics exhibit antimicrobial activity against specific microorganisms, for example, Gram-positive or Gram-negative bacteria, and are most commonly administered orally or intravenously to treat serious infections. Antiseptics are broad-spectrum chemical agents that are safe to use on viable tissues such as skin and mucous membranes but are generally too toxic to be used within the body. Disinfectants are similarly broad-spectrum chemical agents that are widely used on non-viable surfaces for infection control; they are too toxic to be used on or within the body. Some disinfectants may also function as antiseptics at lower, non-toxic concentrations, examples of which are included in Table 1. In health care facilities, disinfectants are routinely used to sanitize non-viable surfaces such as beds, mattresses, trolleys, toilets, bedpans, baths, incubators, ventilators, walls, floors, and ceilings, and to sterilize equipment such as endoscopes.
Microbial tolerance to disinfectants
It is important to bear in mind that different types of microorganisms have different tolerances to antimicrobial agents such as disinfectants. As indicated in Fig. 1, bacterial spores are among the most tolerant and prevalent of microorganisms in health care facilities. The ability of some bacteria to produce spores is of clinical significance because it allows the bacteria to survive under hostile environmental conditions, and the spores will germinate to form actively dividing vegetative/planktonic cells when conditions become more favorable (e.g. within the body). The most important and clinically significant spore-forming bacterium is Clostridioides difficile. C. difficile is found in the gut and can cause conditions ranging from diarrhea to life-threatening C. difficile infection (CDI). The bacteria readily grow in the gut (colon) and are excreted in spore-form, which can then disseminate rapidly throughout a health care facility unless effectively controlled.
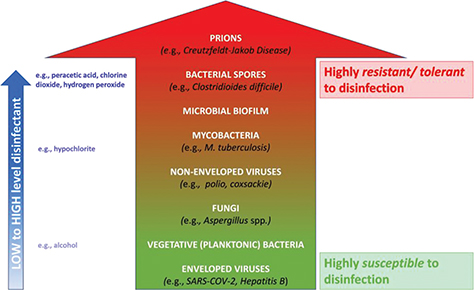
Fig. 1. Microbial tolerance to disinfectants (green = most susceptible; red = most resistant/tolerant). High level disinfectants such as chlorine dioxide, peracetic acid, and hydrogen peroxide are effective against the most resistant/tolerant bacterial forms (i.e. spores, mycobacteria, and biofilm). Note: the most resistant infectious particles (prions) require prolonged sterilization for inactivation.
At the other end of the antimicrobial tolerance spectrum, lipid-enveloped viruses such as SARS-CoV-2 are among the most susceptible to disinfectants (Fig. 1). Since bacteria may be up to 100–1,000 times more tolerant to antimicrobial agents in biofilm form (8), biofilm must be considered as one of the most highly tolerant microbial forms that cannot be overlooked. All vegetative (planktonic) bacteria have the capability to exist in the much more tolerant biofilm form; examples of such pathogens include Staphylococcus aureus, Pseudomonas aeruginosa, and many antibiotic-resistant strains of bacteria.
Bearing in mind that spore-forming bacteria and biofilm bacteria are among the most difficult to treat, disinfectants should ideally provide both sporicidal and anti-biofilm activities.
Requirements for cleaning and disinfection in health care facilities
In 2019, the US Centers for Disease Control and Prevention (CDC) and Infection Control Africa Network (ICAN) published a document entitled ‘Best Practices for Environmental Cleaning in Healthcare Facilities: in Resource-Limited Settings (version 2)’ (16). This document provided information on the ideal properties of disinfectants used for environmental sanitation in health care facilities, and the key requirements are summarized as follows:
- Broad-spectrum antimicrobial activity (i.e. bacteria, spore-forming bacteria, yeasts, fungi, and viruses).
- Rapid and sustained antimicrobial activity (fast acting and maintains residual activity on surfaces).
- Non-irritating to skin or mucous membranes (i.e. non-toxic).
- Maintains activity in the presence of organic matter (e.g. blood), cleaning materials (e.g. cloths), cleaning agents (detergents), and surfaces (e.g. metals and fabrics).
- Environmentally friendly (i.e. does not produce hazardous by-products).
- Cleansing capacity capable of removing dirt, soil, and various organic substances.
- Remains stable in concentrated and diluted (in-use) concentrations.
- Economical and affordable.
- Easy to use.
Simpson et al. (17) also stated ideal criteria for a disinfectant: performance (including activity against biofilm), environment (selective reactivity and non-toxic by-products), safety, and economics (16). Based on these characteristics, chlorine dioxide was rated most highly in comparison with hypochlorous acid, hypobromous acid, and ozone (17).
In Rutala and Weber’s review of disinfection practices in health care facilities, advantages and disadvantages of commonly used disinfectants were listed (Table 2) (18). Despite its widespread use in hospital antisepsis and disinfection, alcohol has limited antimicrobial activity (i.e. not effective against bacterial spores) and exhibits other drawbacks including reduced activity in the presence of organic matter, absence of residual activity, limited activity against non-enveloped viruses, and being flammable. Additionally, both ethanol and isopropyl alcohol have been shown to increase biofilm production in S. aureus and Staphylococcus epidemidis at concentrations ranging from 40 to 95% (19). Sodium hypochlorite (household bleach) is a more effective antimicrobial agent against a broader range of microorganisms (including bacterial spores), but again its activity is compromised by organic matter, it is most effective over a narrow (acidic) pH range, and it has the potential to produce environmentally hazardous by-products and can be corrosive to metals. Quaternary ammonium compounds such as benzalkonium chloride provide a reasonable spectrum of antimicrobial activity, they exhibit detergent properties, and they provide residual activity, but they are ineffective against bacterial spores and non-enveloped viruses, and their activity is compromised by organic matter. Like sodium hypochlorite, peracetic acid and hydrogen peroxide are oxidizing agents with superior characteristics that include a broad spectrum of antimicrobial activity (including bacterial spores), environmentally friendliness (i.e. no hazardous by-products), absence of compromise by organic matter, and surface compatibility.
Another oxidizing agent that has significant advantages over sodium hypochlorite is chlorine dioxide (ClO2). Although ClO2 is a chlorine compound, its chemical behavior is quite different to that of chlorine. The advantages of ClO2 are listed as follows (17, 18) (also see Table 3):
- Environmental impact. Chlorine (Cl2) and ClO2 are both oxidizing agents. However, whereas oxidation by ClO2 occurs only by electron transfer (i.e. specifically with compounds that give up electrons), Cl2 (as a halogen element) halogenates the organic compounds it oxidizes (e.g. carbohydrates, proteins, and fats) to form potentially hazardous by-products such as trihalomethanes (e.g. chloroform) and other halogenated organic compounds that are potentially carcinogenic.
- Selectivity. Cl2 is non-selective and reacts indiscriminately with organic matter. Consequently, Cl2 is rapidly consumed in the presence of organic compounds, and disinfection is compromised until an amount greater than the chlorine demand is available. In contrast, ClO2 is highly selective, has minimal reactivity with organic compounds, and consequently has a significantly greater capacity for disinfection and retention of antimicrobial activity over time. ClO2 has also been shown to be a size-selective disinfectant, inflicting lethal effect on bacterial cells but not on human cells (20).
- pH. The disinfection capacity of ClO2 extends over a wide pH range of between 5 and 10, and its efficiency increases at high pH values. In contrast, sodium hypochlorite (NaOCl) is strongly influenced by pH and has no disinfection capacity above pH 8 (as hypochlorous acid [slightly acidic] transitions to hypochlorite [alkaline]) (17, 21).
- Antimicrobial activity. Both NaOCl and ClO2 exhibit broad spectrum antimicrobial activity (including bacterial spores, biofilm, and non-enveloped viruses), but ClO2 retains its activity in the presence of organic matter (e.g. blood spillages). Since ClO2 is not consumed by organic matter and works over a broader pH range than other chlorine oxidizers, its disinfection capacity is maintained and significantly greater. In a study comparing the efficacy of ClO2 and Cl2 against a variety of bacterial isolates from clinical sources (including methicillin resistant S. aureus, P. aeruginosa, Klebsiella pneumoniae, and Streptococcus pneumoniae), ClO2 was shown to be more effective (22). ClO2 was also shown to be more effective than NaOCl in eliminating coliform bacteria from a variety of surfaces in a hospital out-patient department in Taiwan (23). ClO2 has been reported to eliminate Bacillus cereus spores in biofilm form (24), which represents an extremely stringent situation to demonstrate potency of disinfectant activity. In the food industry and in the disinfection of cooling towers, ClO2 has been widely used due to its excellent biofilm dispersing and bacterial disinfecting properties (17).
- Toxicity. ClO2 is size-selective and is more harmful to bacterial cells than human cells (20).
| Disinfectant Characteristic | Chlorine (Cl) (e.g. sodium hypochlorite (NaOCl)) | Chlorine dioxide (ClO2) | Peracetic acid (PAA) |
| Oxidizing agent (electron receiving) | Yes | Yes | Yes |
| Halogen (salt-producing) chemical element | Yes. Oxidizes by halogenating organic matter. | No. | No |
| Selectivity | Non-selective. Reacts indiscriminately with organic matter, leading to rapid chlorine consumption. Disinfection is subsequently compromised until an amount of chlorine greater than the demand is available (21). | Selective. ClO2 is highly selective and has minimal reactivity with organic matter (only attacks electron-rich bonds in the organic compounds). Consequently, ClO2 has a significantly greater capacity for disinfection and retention of antimicrobial activity over time (17). | Selective and retains activity in the presence of organic matter (18). |
| Broad-spectrum antimicrobial activity | Yes. Effective against bacteria, bacterial spores, biofilm, viruses (enveloped and non-enveloped), yeasts, and fungi. Activity reduced in the presence of organic matter and at alkaline pH. | Yes. Effective against bacteria, bacterial spores, biofilm, viruses (enveloped and non-enveloped), yeasts, and fungi. ClO2 retains its activity in the presence of organic matter (e.g. blood spillages) (21). ClO2 effectively penetrates biofilm and kills associated bacteria (17). ClO2 is size-selective and is more harmful to bacterial cells than human cells (20). | Yes. Effective against bacteria, bacterial spores, mycobacteria, yeasts, fungi, and viruses (18). |
| pH stability | No. Effective at acidic pH (as hypochlorous acid) and loses activity with increasing pH (as hypochlorite) (17, 21). | Yes. Effective over a broad pH range from acidic to alkaline [5–10] (17, 21). | Most effective at pH 6.5–7.5 (25, 26). |
| Environmentally friendly | No. Reacts with organic matter to form potentially hazardous by-products such as trihalomethanes (e.g. chloroform) and other halogenated organic compounds that are potentially carcinogenic (17, 21). | Yes. Minimal reactivity with organic matter and does not halogenate. ClO2 produces harmless by-products (oxygen, sodium chloride, and water) (17, 21). | Yes. Minimal reactivity with organic matter (18). Does not halogenate and produces harmless by-products (acetic acid, oxygen, and water) (16, 27, 28). |
| Green: desirable; yellow: intermediate; pink: undesirable. | |||
Table 3 also includes peracetic acid, another potent oxidizing disinfectant that captures many of the desirable benefits associated with chlorine dioxide, although its optimum activity is confined to a narrow pH range (25, 26).
Conclusions
Bacterial and viral infections are a huge threat to global public health, and today, this is exemplified by the devastation caused by the recent SARS-CoV-2 pandemic and the continuing escalation of bacterial resistance to antibiotics. Infection control and prevention, therefore, become paramount, and disinfectants have a major role to play in our quest to prevent the spread of infections.
This review has highlighted the inadequacies of commonly and widely used disinfectants such as alcohols, hypochlorite, and quaternary ammonium compounds when considering characteristics such as spectrum of antimicrobial activity, surface compatibility, reactivity, stability, and environmental impact. In contrast, oxidizing agents such as peracetic acid and chlorine dioxide are gaining greater acceptance because they meet optimum requirements for disinfectants to a much greater extent than some of the established disinfectants. In particular, chlorine dioxide meets many of the optimum requirements and is unique in its ability to remain effective over a broad pH range.
Given the critical importance of infection control and antimicrobial stewardship in public health and health care facilities today, consideration of chlorine dioxide as a safe, selective, highly effective, and environmentally friendly disinfectant is warranted.
Ethics Approval
Not required.
References
- Zimlichman E, Henderson D, Tamir O, Franz C, Song P, Yamin CK, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US Health Care System. JAMA Intern Med 2013; 173: 2039–46. doi: 10.1001/jamainternmed.2013.9763
- Health Protection Agency. English national point prevalence survey on healthcare-associated infections and antimicrobial use, preliminary data. 2011. Available from: http://webarchive.nationalarchives.gov.uk/20140714085429tf [cited May 2012].
- Guest JF, Keating T, Gould D, Wigglesworth N. Modelling the annual NHS costs and outcomes attributable to healthcare-associated infections in England. BMJ Open 2020; 10: e033367. doi: 10.1136/bmjopen-2019-033367
- Flemming H-C, Wuertz S. Bacteria and archaea on Earth and their abundance in biofilms. Nat Rev Microbiol 2019; 17: 247–60. doi: 10.1038/s41579-019-0158-9
- Wolcott RD. Biofilms cause chronic infections. J Wound Care 2017; 26: 423–5. doi: 10.12968/jowc.2017.26.8.423
- Bowler PG. Antibiotic resistance and biofilm tolerance: a combined threat in the treatment of chronic infections. J Wound Care 2018; 27: 273–7. doi: 10.12968/jowc.2018.27.5.273
- National Institutes of Health Guide: research on microbial biofilms. 2002. Available from: https://grants.nih.gov/grants/guide/pa-files/PA-03-047.html [cited December 2002].
- Høiby N, Ciofu O, Johansen HK, Song Z-J, Moser C, Jensen PØ, et al. The clinical impact of bacterial biofilms. Int J Oral Sci 2011; 3: 55–65. doi: 10.4248/IJOS11026
- Payne AT, Davidson AJ, Kan J, Peipoch M, Bier R, Williamson K. Widespread cryptic viral infections in lotic biofilms. Biofilm 2020; 2: 100016. doi: 10.1016/j.bioflm.2019.100016
- Besharati S, Farnia P, Farnia P, Ghanavi J, Velayati AA. Investigation of the hypothesis of biofilm formation in coronavirus (COVID‑19). Biomed Biotechnol Res J 2020; 4: S99–100.
- Voigt AM, Faerber HA, Wilbring G, Skutlarek D, Felder C, Mahn R, et al. The occurrence of antimicrobial substances in toilet, sink and shower drainpipes of clinical units: a neglected source of antibiotic residues. Int J Hyg Environ Health 2019; 333: 455–67. doi: 10.1016/j.ijheh.2018.12.013
- Flemming H-C, Wingender J, Szewwzyk U, Steinberg P, Rice SA, Kjelleberg S. Biofilms: an emergent form of bacterial life. Nat Rev Microbiol 2016; 14: 563–75. doi: 10.1038/nrmicro.2016.94
- Bowler PG, Murphy C, Wolcott RD. Biofilm exacerbates antimicrobial resistance: is this a current oversight in antimicrobial stewardship? Antimicrob Resist Infect Control 2020; 9: 162. doi: 10.1186/s13756-020-00830-6
- Hu H, Johani K, Gosbell IB, Jacombs AS, Almatroudi A, Whiteley GS, et al. Intensive care unit environmental surfaces are contaminated by multidrug-resistant bacteria in biofilms: combined results of conventional culture, pyrosequencing, scanning electron microscopy, and confocal laser microscopy. J Hosp Infect 2015; 91: 35–44. doi: 10.1016/j.jhin.2015.05.016
- Ledwoch K, Dancer SJ, Otter JA, Kerr K, Roposte D, Rushton L, et al. Beware biofilm! Dry biofilms containing bacterial pathogens on multiple healthcare surfaces; a multicentre study. J Hosp Infect 2018; 100: e47–56. doi: 10.1016/j.jhin.2018.06.028
- Centers for Disease Control and Prevention and Infection Control Africa Network. Best practices for environmental cleaning in healthcare facilities in resource-limited settings. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; Cape Town, South Africa: Infection Control Africa Network; 2019. Available from: https://www.cdc.gov/hai/pdfs/resource-limited/environmental-cleaning-RLS-H.pdf and http://www.icanetwork.co.za/icanguideline2019/.
- Simpson GD, Miller RF, Laxton GD, Clements WR. A focus on chlorine dioxide: the ‘ideal’ biocide. Corrosion 93. New Orleans, LA; March 1993. Available from: http://www.clo2.gr/en/pdf/secure/chlorinedioxideidealbiocide.pdf. [cited March 1993].
- Rutala WA, Weber DJ. Disinfection and sterilization in healthcare facilities. Infect Dis Clin N Am 2016; 30: 609–37. doi: 10.1016/j.idc.2016.04.002
- Luther K, Bilida S, Mermel LA, LaPlante KL. Ethanol and isopropyl alcohol exposure increase biofilm formation in Staphylococcus aureus and Staphylococcus epidermidis. Infect Dis Ther 2015; 4: 219–26. doi: 10.1007/s40121-015-0065-y
- Noszticzius Z, Wittmann M, Kály-Kullai K, Beregvári Z, Kiss I, Rosivall L, et al. Chlorine dioxide is a size-selective antimicrobial agent. PLoS One 2013; 8: e79157. doi: 10.1371/journal.pone.0079157
- Performacide: the advantage of chlorine dioxide – performacide. 2021. https://www.performacide.com/the-science.
- Al-Sa’ady AT, Nahar H, Saffah FF. Antibacterial activities of chlorine gas and chlorine dioxide gas against some pathogenic bacteria. Eurasia J Biosci 2020; 4: 3875–82.
- Lin KS, Hsieh MJ, Liou MJ, Lee SL, Lai C-K. Disinfection effect of chlorine dioxide on air quality control in Armed Forces General Hospital of Taiwan. Nat Sci 2007; 5: 94–9.
- Nam H, Seo H-S, Bang J, Kim H, Beuchat LR, Ryo J-H. Efficacy of gaseous chlorine dioxide in inactivating Bacillus cereus spores attached to and in a biofilm on stainless steel. Int J Food Microbiol 2014; 188: 122–7. doi: 10.1016/j.ijfoodmicro.2014.07.009
- Dunkin N, Coulter C, Weng SC, Jacangelo JG, Schwab KJ. Effects of pH variability on peracetic acid reduction of human norovirus GI, GII RNA, and infectivity plus RNA reduction of selected surrogates. Food Environ Virol 2019; 11: 76–89. doi: 10.1007/s12560-018-9359-z
- McFadden M, Loconsole J, Schockling AJ, Nerenberg R, Pavissich JP. Comparing peracetic acid and hypochlorite for disinfection of combined sewer overflows: effects of suspended-solids and pH. Sci Tot Environ 2017; 599–600: 533–9. doi: 10.1016/j.scitotenv.2017.04.179
- Kim J, Huang C-H. Reactivity of peracetic acid with organic compounds: a critical review. ACS EST Water 2021; 1: 15−33. doi: 10.1021/acsestwater.0c00029
- Steiner N. Evaluation of peracetic acid as an environmentally safe alternative for hypochlorite. Textile Chem Color 1995; 27: 29–32.